Juan Estaun Panzano
Extracellular space in neurodegenerative disorders
décembre 2024 Directeur(s) de thèse : Erwan Bezard Résumé de thèseThe extracellular space (ECS), comprising the narrow compartment between the cells filled with interstitial fluid and the extracellular matrix (ECM) it is key for communication and transport between brain cells and is vital for maintaining brain homeostasis. In this work, we try to shed some light on the potential alterations of the ECS in the proteinopathies context, particularly synucleinopathy and amyloid pathology. These protein accumulations are known to disrupt normal brain functions, but their impact on the brain’s ECS, which plays a critical role in molecular/protein diffusion and waste clearance, has not been thoroughly examined.
In the introduction, I present an overview of amyloid and α-synuclein pathology. Later, ECS and its components will be introduced, focusing on the extracellular matrix and diffusion processes. Furthermore, the glymphatic system, a proposed waste-clearance mechanism linked to the ECS, is discussed. Understanding these mechanisms is crucial for interpreting how the ECS is altered in proteinopathies and its repercussions. Additionally, the study reviews current techniques used to investigate the ECS, including traditional methods, advanced imaging, and single-particle tracking techniques.
I then present the structural and rheological changes in the ECS in two different models of proteinopathies, focusing on the altered diffusion properties within this space and its implications for disease pathology. A variety of advanced techniques were employed to address these questions. In the first sub-project, we report ECS diffusivity changes in the striatum of a mouse synucleinopathy model. Using single-walled carbon nanotube (SWCNTs) tracking in the ECS, the study demonstrates that intracellular α-synuclein assemblies can significantly alter nanoscale diffusion in the striatal ECS.
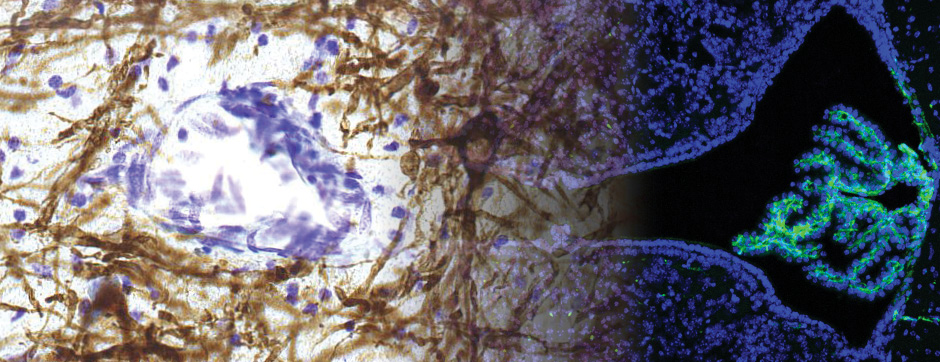
We repeat the same approach in an amyloid mouse model (APP/PS1), with special focus on diffusion alterations around amyloid plaques and its fine relationship with matrix disruptions. We employed a complementary set of nanoscopic imaging techniques to investigate the ECS alterations: Two-photon shadow imaging in vivo and ex vivo, revealed cortical amyloid as a dense ring of cells and a central core. Quantum dot SPT tracking unraveled the core of the plaque is not easily penetrable by ab-sized molecules. Furthermore, we report ECS rheological parameters are heterogeneous in and around plaques, with an increased diffusivity in the amyloid animal and low nanoparticle density in the core. Using SWCNTs, we confirmed these altered local diffusion properties in the cortex of AD mice, with an overall higher local diffusivity in APP/PS1 cortex. We found an altered ECM, notably disrupted within the amyloid plaque but not only, providing a rationale for the altered rheological dynamics in AD brain tissue and shedding new light on strategies to develop effective Aβ plaques-penetrating therapies.
Overall, the findings presented in this work contribute to a deeper understanding of the ECS’s role and changes in neurodegenerative diseases. The results suggest that altered diffusion of the brain ECS are a relatively common phenomenon. Future modelling studies of disease expansion or therapeutics design should considerthese changes.