Morgane Darricau
Pathophysiology of tauopathies and modeling in non-human primate
novembre 2023 Directeur(s) de thèse : Vincent Planche Résumé de thèseMy PhD project was dedicated to the study of tauopathies. Tauopathies are a group of neurodegenerative diseases characterized by the accumulation and aggregation of pathological misfolded tau proteins. Alzheimer’s disease (AD), progressive supranuclear palsy (PSP), cortico-basal degeneration (CBD) and some frontotemporal lobar degenerations (FTLD) are part of the large family of tauopathies. These pathologies differ both clinically and anatomically, with a distinct anatomical distribution of typical lesions.
However, the mechanisms of initiation and progression of tauopathies remain poorly understood for these pathologies. Recent studies have suggested that the development of tauopathies is based on “prion-like” features, where a single tau proteopathic seed would have the ability to transmit pathogenic information, to “contaminate” non-pathological soluble tau proteins leading to neurotoxicité. Tau aggregates would be transmitted from cell to cell, thus explaining the spreading of tauopathy in different connected brain regions. However, this hypothesis is based only on in vitro and vivo rodents experiments. These experimental models offer many advantages for the study of tauopathies, but they do not fully reproduce human pathologies. In order to develop relevant pre-clinical studies of these pathologies, it seems necessary today to work on experimental models as close as possible to human models.
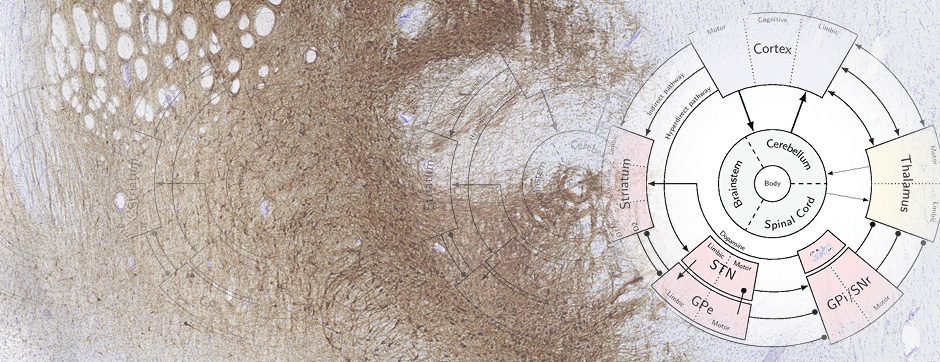
Thus, in my PhD project, we proposed to model tauopathies in rhesus macaques (Macaca mulatta), an animal phylogenetically closest to humans. We focused on two tauopathies: PSP and Alzheimer’s disease.
The main aim of my PhD was to demonstrate the “prion-like” propagation of these two tauopathies in non-human primates. We focused on two tauopathies : PSP and Alzheimer’s disease.
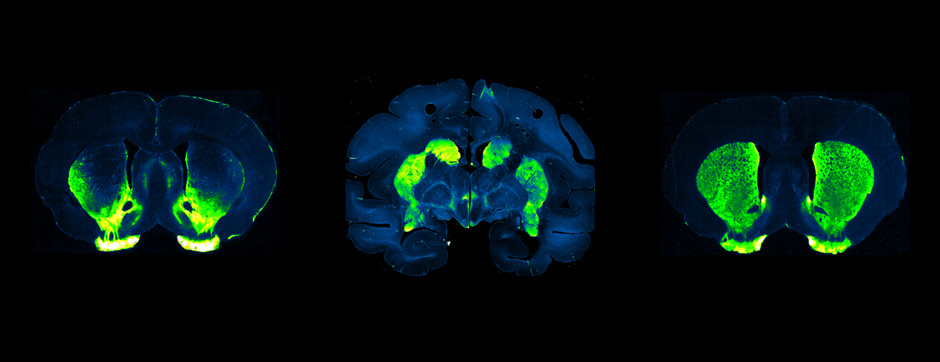
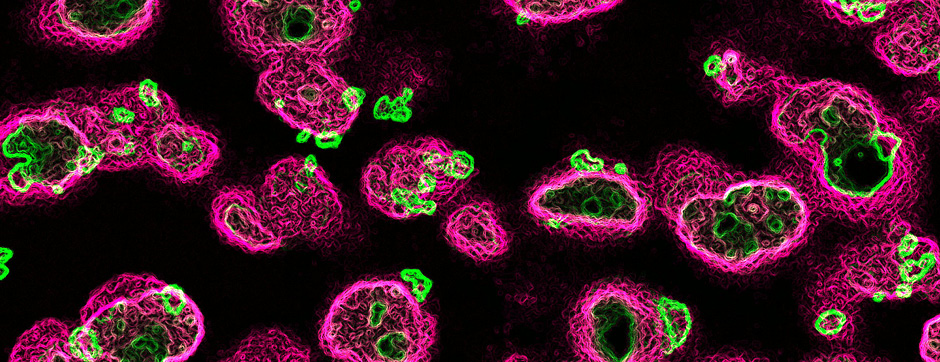
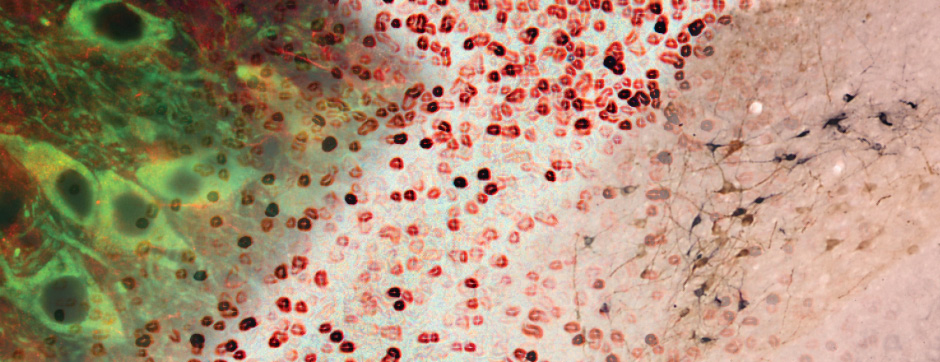
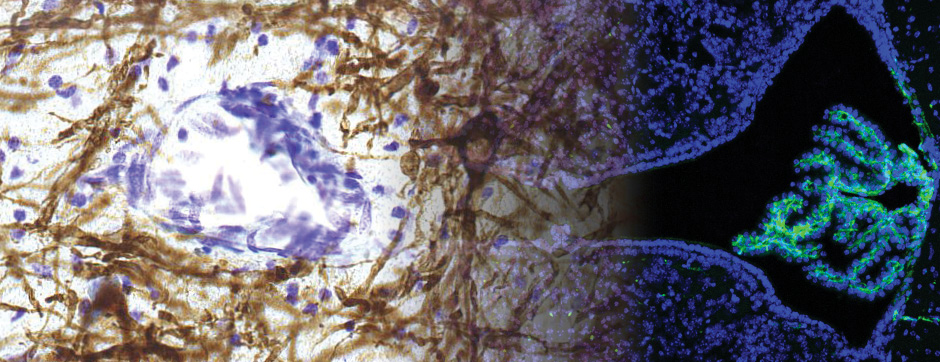
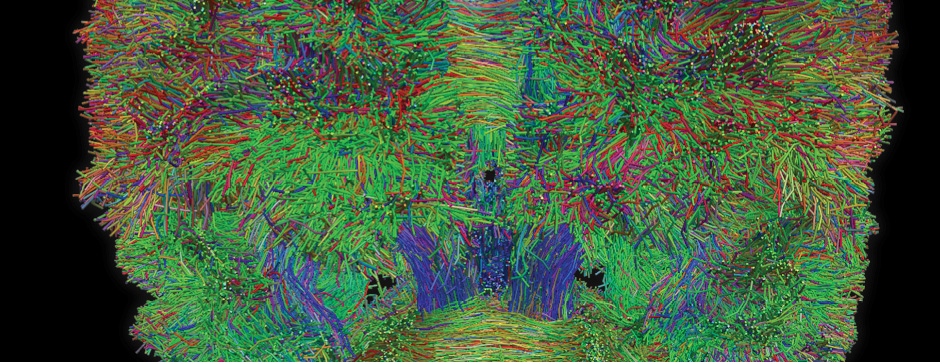
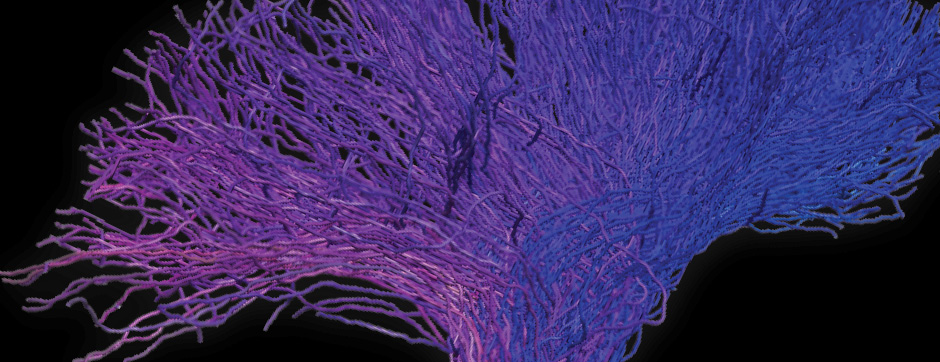
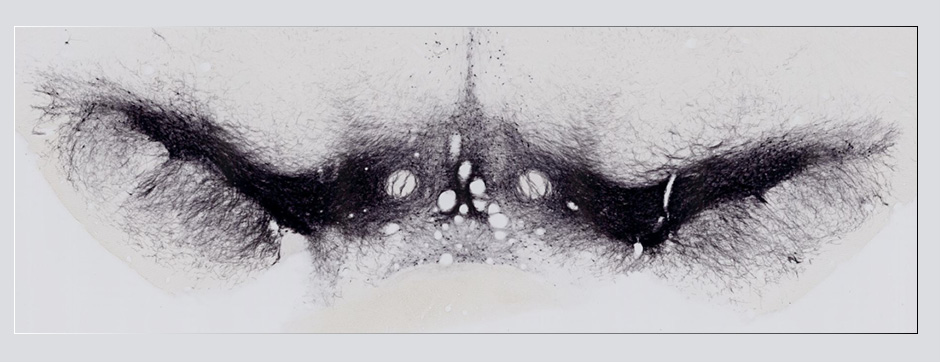
Using intracerebral injections of tau proetopathic seeds extracted and purified from PSP and Alzheimer’s disease patients, we induced the pathologies. Indeed, we were able to observe in these primates the presence of lesions typical of these two tauopathies, inclusions of hyperphosphorylated tau protein in neurons and glial cells for PSP, and lesions of NFTs and neuropils threads for Alzheimer’s disease. The observation of these lesions around injection sites and within connected structures suggests the “prion-like” feature of tau protein, where the simple injection of pathogenic material allows the formation of new aggregates progressing from cell to cell.
These various projects have enabled us to better understand the pathophysiology and mechanisms involved in the cognitive and motor disorders of these tauopathies. Furthermore, the development of an animal model close to humans pave the way to new therapies.