Eduarda Gervini Zampieri Centeno
The investigation of songbirds’ cortical-basal ganglia neural sleep dynamics through an Open Science-inspired computational framework
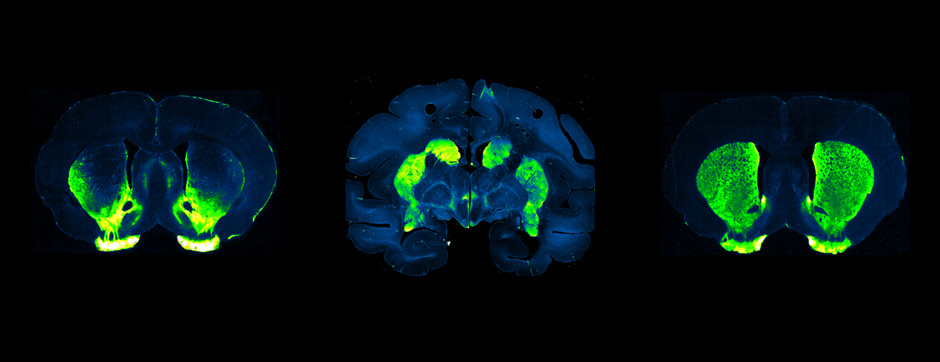
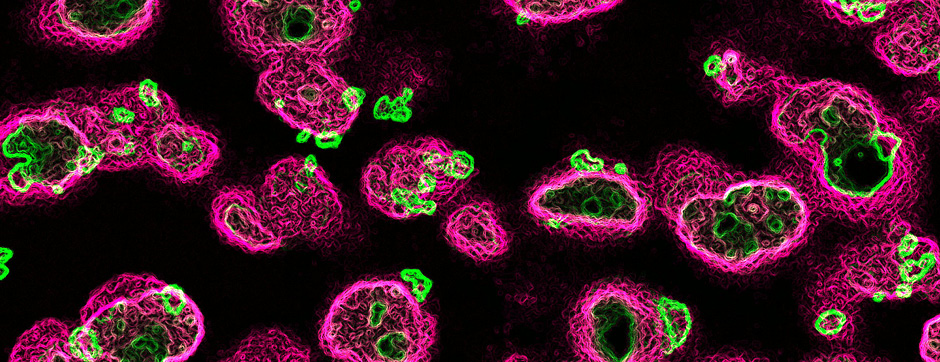
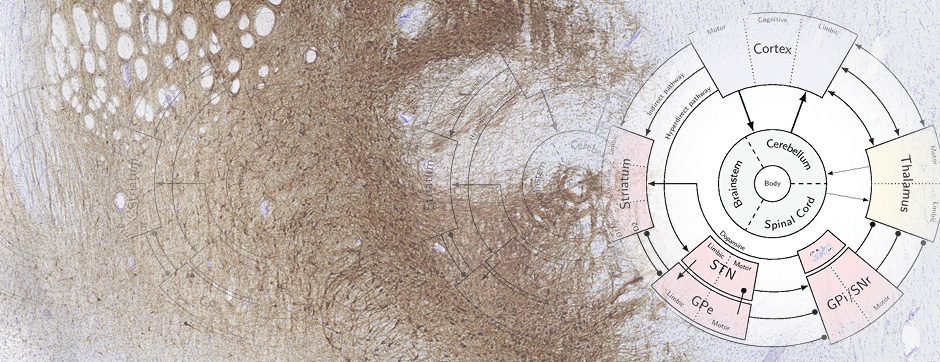
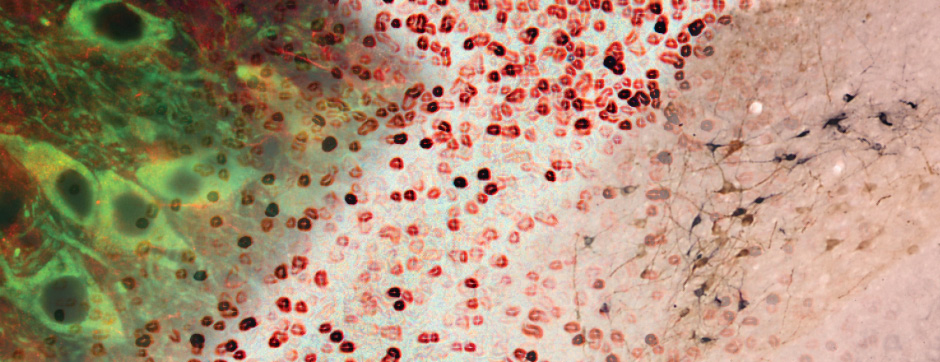
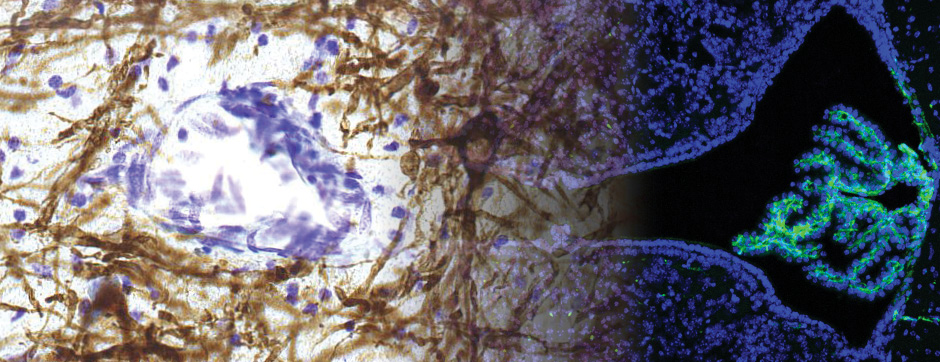
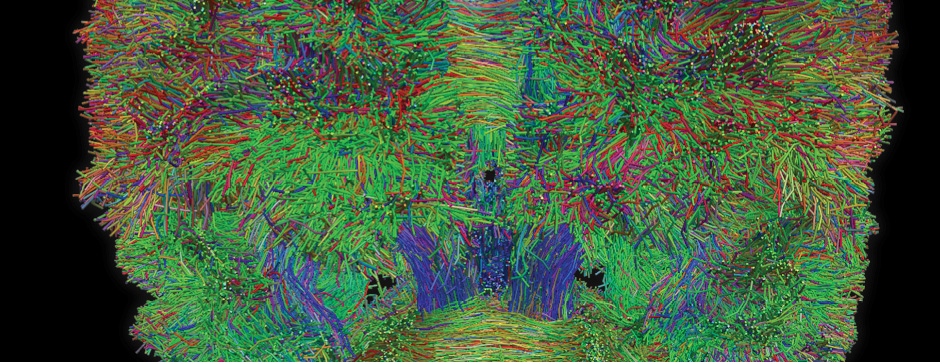
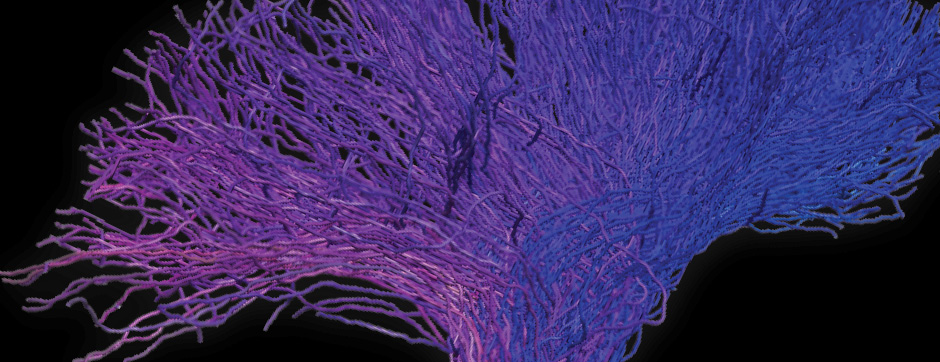
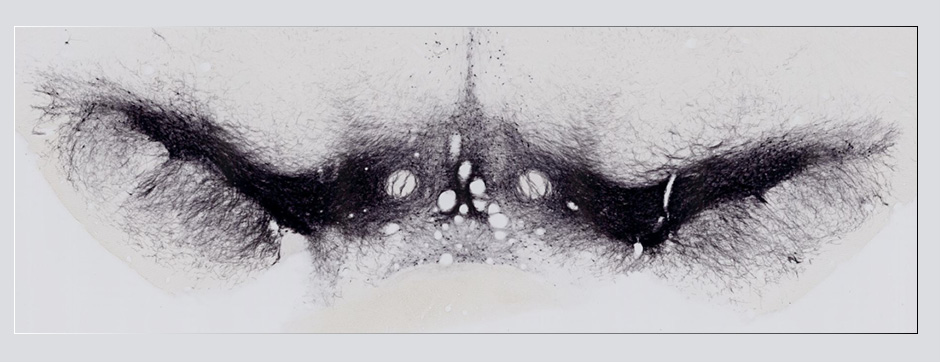
novembre 2024 Directeur(s) de thèse : Arthur Leblois Résumé de thèseAs neuroscience incorporates big data and advanced technologies, it becomes crucial to prioritise data management, reproducibility, and resource sharing. Open Science (OS) offers a framework to fulfil these objectives. Our project utilizes OS principles to explore the neural mechanisms underlying vocal learning in songbirds—a well-established model for studying speech learning. Similar to how humans learn how to speak, birdsong acquisition relies on a long trial-and-error auditory-feedback-dependent process engaging cortical and subcortical areas in the brain. Mastering such a complex sensorimotor skill is no small feat, and songbirds have a dedicated brain circuitry for acquiring songs, which includes a cortical-basal-ganglia (CBG) loop homologous to that found in mammals. Several studies have demonstrated how this CBG loop is necessary for song acquisition and plasticity. However, the process through which learning is consolidated within this loop remains unknown. We know that successful song learning depends on sleep and that, during the night, oscillatory activity emerges and neural activity related to singing is replayed in parts of the song circuitry. Could neural oscillations and replays underlie song consolidation in the avian CBG loop? Drawing from the understanding of how synchronised oscillatory activity in the hippocampal-cortical network facilitates the transfer and consolidation of memories, we used Neuropixel 1.0 probes in the avian CBG loop to explore whether similar neural mechanisms can be observed, suggesting participation in song consolidation. To efficiently handle our high-yield and multiplexed datasets (electrophysiology, behaviour, and histology), we first built the necessary computational infrastructure with standardised (meta)data management and sharing protocols, automated spike sorting, and data-tailored visualisation and analysis tools. Then, we applied this toolset to sleep recordings from seven birds and were the first to identify that avian CBG-loop cortical areas exhibit higher local field coherence, neural firing variability, and spike-to-field entrainment during slow wave sleep, as compared to the basal ganglia, which instead had a more desynchronised and uncoordinated profile.